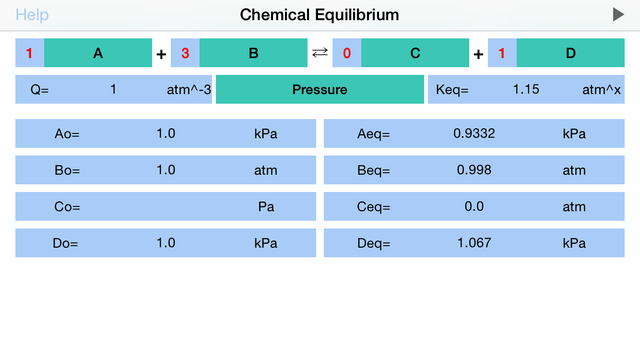
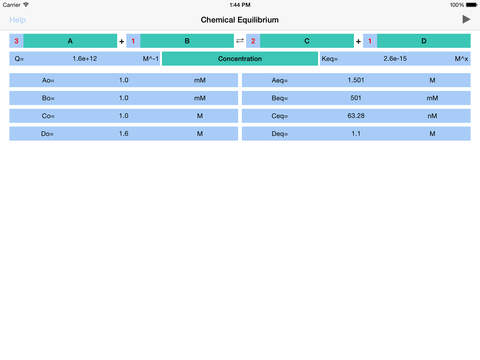
Chemical Equilibrium is a tool for accurate estimation of concentrations and pressures of the chemical reaction reactants and products at equilibrium. The application derives final, equilibrium parameters based on equilibrium constants (Kc and Kp), initial concentrations and stoichiometric coefficients. Additionally, app calculates reaction quotient for any given concentrations and stoichiometry.For a general chemical reaction: aA + bB = cC + dD,the Reaction Quotient (Q) is defined by: Q= (C^c * D^d) / (A^a * B^b) where A, B, C, D – are compounds’ concentrations or pressures and a, b, c, d are stoichiometric coefficient*. Sum of powers determines the reaction order. *Importantly, real system powers may differ from reaction coefficients, due to overall reaction mechanism complexity. If Q is not equal to reaction equilibrium constant K (Kc for concentrations and Kp for pressures), then reaction is not at equilibrium and it will proceed (due to difference in forward and reverse reaction speeds) to the direction defined by Q.If Q is less than K reaction will move towards products (C and D), in opposite case – reverse reaction will prevail.Example:For given reaction: 2A + 1B = 3C + 2D , initial reaction concentrations are Ao=0.1M, Bo=0.2M, Co=0.3M and Do=0.4M and Kc=1 M^2. the Reaction Quotient (Q) is defined by: Q= (C^3 * D^2) / (A^2 * B) Loading the data to “Chemical Equilibrium” app, Reaction Quotient is immediately calculated, giving Q= 2.16 M^2. Apparently Q>K, suggesting that reaction is too close to the products side and reverse reaction will prevail to bring the reaction back to the equilibrium. The new equilibrium concentrations of reaction components can be derived from the equation:Apparently, solving this equation is rather demanding task, that turns to be unnecessary, since “Chemical Equilibrium” app immediately returns the answer: Aeq=0.1185M, Beq=0.2092M, Ceq=0.2723M and Deq=0.3815M.The application features:•To start calculation user is required to fill in stoichiometric coefficients, initial concentrations (pressures) of available components, and equilibrium constant Kc (Kp).•Reaction coefficients are actually representing powers and therefore define reaction order.•To start calculation initially or after updating the concentration or coefficient fields, user is requested to tap Run button!•Significant attention should be paid to concentration, pressure and equilibrium constant units. App automatically changes the set of available units as per order of reaction!•The power of equilibrium constant units depicted as “x” is defined as (c+d-a-b), where a, b, c and d are the stoichiometric coefficients of the reaction. •The basic conversions are as follows: 1 M= (1e3) mM1 atm=101325 Pa
免費玩Chemical Equilibrium APP玩免費
免費玩Chemical Equilibrium App
| 熱門國家 | 系統支援 | 版本 | 費用 | APP評分 | 上架日期 | 更新日期 |
|---|---|---|---|---|---|---|
| 未知 | iOS App Store | 1.0 App下載 | $1.99 | 2015-04-04 | 2015-06-04 |